Ocean Corrosion and the Dissolution of WWII Shipwrecks

Header image shows USS Quincy, as explored by ROV Hercules in July, 2025, with evidence of corrosion.
What is happening to the over 20,000 ships from World War II (WWII) that have lain at the bottom of the ocean for more than half a century? Over eight decades, these steel hulls stand as memorials to a global conflict. But as they slowly become rusting giants, these historical relics are also slow-motion chemistry experiments. Understanding how they continue to change is essential, as vessels sometimes contain hazardous materials, such as fuel or oil, with the potential for environmental damage. So, how exactly does chemistry cause a ship made of iron and steel to fall apart on the bottom of the sea?
When a ship’s iron reacts with the ocean’s oxygen, it creates iron oxide, Fe2O3 (commonly known as rust). This flaky, reddish-brown material weakens the metal over time in a process known as corrosion.
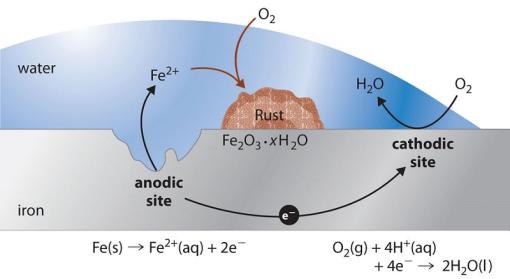
The four required components of a corrosion reaction are an anode, a cathode, a conductor path, and a conducting electrolyte.
- The anode is the electrode (aka conductor) that donates electrons and into which current flows, becoming corroded.
- The cathode is the electrode to which electrons are transferred.
- The conductor path is the physical contact between the anode and cathode.
- The electrolyte is a medium that allows the movement of ions between the anode and cathode. It can be a liquid solution, such as saltwater or soil, or even humid air.
For steel shipwrecks, the anode, iron, gives electrons away, and the cathode, dissolved oxygen, receives those electrons. The dissolved salts in seawater act as electrolytes, enhancing the conductivity of the water and speeding up this electron transfer process. In this electrochemical reaction, known as galvanic corrosion, the ship’s metal is essentially returning to its natural state as an ore, causing the metal to disintegrate.
How does corrosion under the sea work?
Ocean conditions accelerate the corrosion process. The ocean is (on average) 3.5% salt by weight, mainly sodium chloride (NaCl). This makes seawater a much better conductor of electricity than freshwater, allowing for faster and easier chemical reactions as salt ions facilitate the movement of electrons in solution. Different ocean conditions can alter the pace of corrosion. Warmer temperatures make atoms interact more vigorously, which increases the speed of chemical reactions, including corrosion. Higher pressure also increases reaction speed. The more dissolved oxygen in a body of water, the more readily available the cathode is to accelerate rusting. Higher salinity water provides more electrolytes for the corrosion reaction. Strong ocean currents also bring a continuous supply of reactants, speeding up corrosion. Conversely, life growing over a surface can reduce the speed of corrosion by limiting the metal’s exposure to electrolytes.
In the deep sea, corrosion processes are still being studied to understand how factors interact in the combined low temperature, high pressure, and low oxygen conditions.
Another type of ocean corrosion comes from the presence of bacteria. Some tiny microbes live on metallic surfaces and can accelerate decay by expelling acids or otherwise changing an area’s chemistry. Scientists refer to this process as biocorrosion or microbiologically influenced corrosion (MIC). The MIC process can decompose metal hulls even faster than electrochemical corrosion, accelerating degradation of sites before archeologists or scientists can fully study the wreck and corrosion interactions.
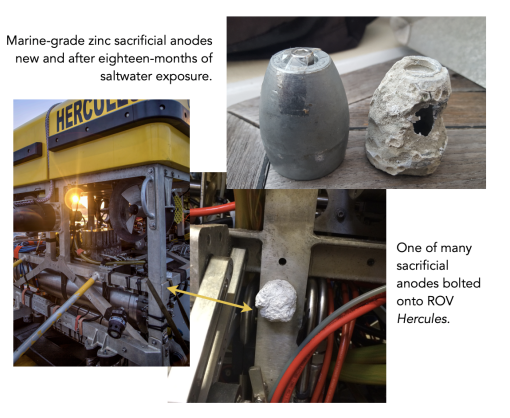
Preventing corrosion: What are sacrificial anodes?
E/V Nautilus explores with two remotely operated vehicles (ROVs), Hercules and Atalanta, which scientists use to study deep ocean environments. The frames of both vehicles are made of stainless steel, which is highly resistant to corrosion. However, if left alone would eventually be impacted by the salty water of the sea. To reduce corrosion damage on the ROVs’ frames, engineers bolt on something called a sacrificial anode. These (usually zinc) metallic alloys, shaped into disks or blocks, are highly electrically active, meaning they “donate” electrons into solution rapidly, chemically outcompeting the steel frame as a reaction anode. By bolting these anodes all over the ROVs, the protection is spread into many conductor paths as they “sacrifice” themselves to protect important vehicle infrastructure.
What happens to shipwrecks in the ocean?
Understanding the impacts of corrosion on ships begins with understanding the construction materials. Ancient wooden ships naturally decompose in the ocean when there is enough oxygen present for biodegrading organisms like bacteria and worms. When explorers locate these sites, typically the wooden portions of a vessel are gone, but non-biodegradable materials are left behind. In ancient Mediterranean ships, ceramic amphorae outlined the final resting place of ancient wooden shipwreck sites.
When we’re considering ships made of metal, a deeper understanding of material science becomes key to studying corrosion impacts. Different alloys of steel corrode at different rates. A metal alloy is a substance composed of two or more elements, at least one of which is a metal, designed to enhance specific properties like strength, durability, or corrosion resistance. Additionally, the many varied parts of ships are made of many different metal alloys, including less-resistant, lower-quality steel, being used in construction due to wartime shortages.
Examining how corrosion is impacting different ship components, like deck plating, superstructure, armored belt panels, armaments, can help researchers understand specific conditions on the seafloor. During the Maritime Archaeology of Guadalcanal expedition, part of the evaluation of site conditions will be to study corrosion to understand how degradation could lead to potential sources of marine pollution.
Several key factors determine how quickly a metal shipwreck corrodes:
- Depth: Shallow wrecks are exposed to more oxygen and warmer temperatures, which can increase corrosion. On the flip side, deeper wrecks have less oxygen but face higher pressure and often more microbial activity.
- Temperature: Warmer waters typically speed up chemical reactions, including rust formation. Tropical waters are often shallower and warmer, seeing faster rates of corrosion.
- Salinity: More dissolved salt generally means faster corrosion.
- Biology: Certain bacteria and marine organisms attach to the wreck and contribute to increased corrosion or degradation of the vessel.
- Exposure: Fast currents can remove protective layers of rust and expose fresh pieces of metal to the water. On the other hand, sediment can sometimes bury parts of the wreck, protecting them.
- Original Condition: In order to combat corrosion, many vessels are covered with anti-fouling paints to deter biological growth on the hull and limit overall exposure of metal to seawater. In WWII, oil-based paints were also used for corrosion protection, but had limited duration and introduced the risks of flammability.

Submarine USS Bugara as recorded by OET in 2017.
How does this impact World War II wrecks specifically?
In the book, Threats to Our Ocean Heritage: Potentially Polluting Wrecks (Brennan et al, 2024) authors reveal that over 3,800 ships were lost during WWII in the Pacific Region. “At the time of their loss, these ships contained as many as 1.5 billion gallons of petrochemicals, and hundreds of thousands of tons of explosive ordnance, an unknown amount of which remains in these wrecks today,” they say.
Researchers estimate that during their eighty years of exposure to the corrosive environment of the Iron Bottom Sound, the ships “experienced a corrosion rate of approximately 0.1 mm per year, putting them at an increasing risk of structural failure annually.” This measurement is a rough estimate that doesn’t take into account the additional factors discussed earlier in this blog.
“The ongoing corrosion of these potentially-polluting wrecks represents a critical challenge in the sustainable management of these sites, as the wrecks are steadily approaching inevitable collapse, releasing any remaining pollutants they contain,” says Brennan, et al (2024).
Understanding corrosion as an often unseen, though omnipresent, natural process is key to managing shipwrecks that are both unchanging historical memorials and ever-changing parts of the ocean landscape. Due to high rates of corrosion, the clock is ticking.

Maritime Archaeology of Guadalcanal: Iron Bottom Sound
Located in the Solomon Islands between the islands of Guadalcanal, Savo, and Nggela, Iron Bottom Sound was the stage of five major naval battles between August and December 1942 which resulted in the loss of over 20,000 lives, 111 naval vessels, and 1,450 planes. These underwater cultural heritage sites now rest on the seafloor offshore Honiara in a confined area less than 25 nautical miles wide, 40 nautical miles long, and 1,400 meters deep.



